Abstract
INTRODUCTION: Luxeptinib (CG-806) is an orally active, non-covalent inhibitor of BTK and FLT3 kinases. It suppresses BCR signaling pathways (LYN, SYK, BTK, AKT, ERK), MAP kinases, and other oncogenic pathways in cell lines and primary leukemic cells. Luxeptinib kills malignant B-cells insensitive to ibrutinib or venetoclax with concentrations in the nanomolar range and shows enhanced activity in combination with venetoclax. Luxeptinib is currently being evaluated in a Phase 1a/b trial in patients with relapsed or refractory B-cell malignancies (NCT03893682).
AIMS: Primary objectives are to assess the safety and tolerability of luxeptinib and determine the recommended phase 2 dose for future clinical trials in patients with R/R CLL/SLL or B-NHL. Secondary objectives include elucidation of pharmacokinetics (PK) and evidence of antitumor activity.
METHODS: Luxeptinib is administered continuously as oral capsules BID in 28-day cycles, in ascending cohorts of 3 patients. Treatment emergent adverse events (TEAEs) and tumor responses are evaluated per disease specific guidelines.
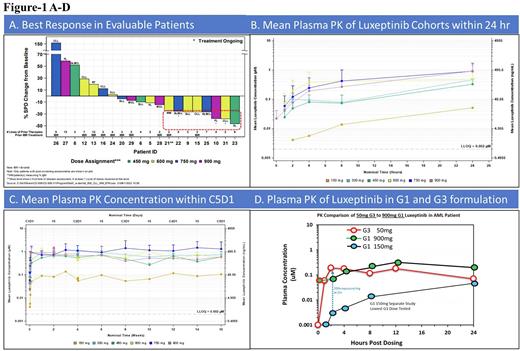
RESULTS: As of June 30, 2022, a total of 35 patients (median age 62 years, 23 male and 12 female, 16 CLL/SLL, 7 follicular lymphoma (FL), 1 Richter's transformation lymphoma, 1 marginal zone lymphoma (MZL), 2 Waldenstrom macroglobulinemia (WM), 4 mantle cell lymphoma (MCL), 4 diffuse large B cell lymphoma (DLBCL)) with a median of 3 prior systemic regimens (range 1 - 13) have been treated with luxeptinib at doses of 150 mg (n=1), 300 mg (n=1), 450 mg (n=10), 600 mg (n=7), 750 mg (n=9), and 900 mg (n=7). Drug related grade ≥ 3 TEAEs include decreased neutrophil count (n=6, 17.1%), decreased white blood cell count, decreased platelet count, diarrhea, anemia, and leukocytosis (n=2, 5.7% each), and increased ALT, headache, neutropenia, hyperhidrosis, hypertension, and neutropenic sepsis (n=1, 2.9% each). One patient experienced a new onset grade 2 hypertension during screening (prior to treatment with luxeptinib) that increased to grade 4 after beginning luxeptinib treatment at 750 mg BID for 5 days. No drug-related hypertension was observed in any other patient treated to date and retrospective analysis of data and patient history suggest the hypertension was not attributable to luxeptinib. PK analysis shows 750 mg BID cohorts have plasma level of luxeptinib > 1µM average steady-state (Cmin) that is sustained over multiple cycles. Response evaluations were available for 20 patients who had at least one imaging study or IgM measurement (WM patients) after starting treatment. Thirteen (3 SLL, 3 FL, 2 CLL, 1 WM, 2 MCL, and 2 DLBCL) out of 20 evaluable patients had various reductions of lesion size or IgM measurements compared to baseline, demonstrating anti-tumor activity. FDG PET-CT scans in 3 FL patients, each treated with at least 2 prior regimens, experienced tumor growth after starting luxeptinib, but demonstrated reductions in lesion size below both peak and pre-treatment baseline tumor sizes after increasing their luxeptinib dose to 600 mg BID. Similarly, additional patients with other NHL (WM, MCL & DLBCL) and SLL/CLL with prior multiple regimens including Ibrutinib, experienced tumor reduction below baseline. Doses levels up to and including 900 mg BID were cleared without additional DLTs. Because increasing the dose to 900 mg BID did not result in substantially greater PK exposure, a novel formulation (G3) of luxeptinib was developed to increase bioavailability. In pre-clinical animal studies this demonstrated 6-30-fold greater exposure compared to the original formulation. Single dose testing in patients produced more rapid and greater per mg exposures than the original formulation. The G3 formulation had 200 times greater exposure/mg at 2 hr when comparing single doses of G3 at 50 mg to 150 mg of the original formulation. Computational modeling of single dose PK data is in progress to inform the potential for continuous dosing with G3 to reduce pill burden and deliver greater exposure and anti-tumor activity.
CONCLUSIONS: Luxeptinib has a favorable safety profile in patients treated with 150 mg to 900 mg BID over multiple cycles. Antitumor activity was observed in multiple B-NHL subtypes and CLL/SLL patients post ibrutinib relapse. Enrollment of additional patients at dose level 6 (900 mg) is ongoing while the more bioavailable G3 formulation is being explored. Updated clinical data will be presented at the meeting.
Disclosures
Samaniego:TG Therapeutics: Honoraria. Villasboas:Aptose: Research Funding; CRISPR: Research Funding; Enterome: Research Funding; Epizyme: Research Funding; Kite Pharma: Research Funding; Regeneron: Research Funding. Burke:Roche/Genentech: Consultancy; Morphosys: Consultancy; Kymera: Consultancy; Nurix: Consultancy; Kura: Consultancy; Epizyme: Consultancy; Bristol Myers Squibbs: Consultancy; BeiGene: Consultancy, Speakers Bureau; AstraZeneca: Consultancy; Adaptive Biotechnologies: Consultancy; Abbvie: Consultancy; SeaGen: Consultancy, Speakers Bureau; TG Therapeutics: Consultancy; Verastem: Consultancy; X4 Pharmaceuticals: Consultancy. Reid:ADC Therapeutics: Research Funding; Aptose: Research Funding; Millennium: Research Funding; Xencor: Research Funding; Epicentrx: Other: Spouse is employed by epicentrx. Cherry:BMS: Other: Ad Board. Melear:AstraZeneca: Speakers Bureau; Janssen: Speakers Bureau; TG Therapeutics: Speakers Bureau. Cobb:Merck & Co., Inc.: Research Funding. Conkling:Ontada: Current Employment; Aposte: Research Funding; Merck: Research Funding; Bristol Myers Squibb: Research Funding; Pfizer: Research Funding. Roeker:AbbVie: Consultancy, Divested equity in a private or publicly-traded company in the past 24 months; AstraZeneca: Consultancy; Janssen: Consultancy; Loxo Oncology: Consultancy, Other: Travel support, Research Funding; Pharmacyclics: Consultancy; Pfizer: Consultancy, Research Funding; Beigene: Consultancy; TG Therapeutics: Consultancy; Abbott Laboratories: Current equity holder in publicly-traded company; Aptose Biosciences: Research Funding; Ascentage: Consultancy; Qilu Puget Sound Biotherapeutics: Research Funding. Hu:Aptose Biosciences: Current Employment, Current equity holder in publicly-traded company. Sinha:Aptose Bioscience: Current Employment, Current holder of stock options in a privately-held company. Rice:Aptose Biosciences: Current Employment, Current equity holder in publicly-traded company, Membership on an entity's Board of Directors or advisory committees, Patents & Royalties: Author on patents. Bejar:Aptose Biosciences: Current Employment, Current equity holder in publicly-traded company; Gilead: Other: data safety monitoring committees chair; Epizyme: Other: data safety monitoring committee chair; BMS: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Takeda: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal